“The Epicurean selects the situations, the persons and even the events which suit his extremely sensitive, intellectual constitution; he renounces the rest — that is to say, by far the greater part of experience — because it would be too strong and too heavy fare for him. The Stoic, on the contrary, accustoms himself to swallow stones and vermin, glass-splinters and scorpions, without feeling any disgust: his stomach is meant to become indifferent in the end to all that the accidents of existence cast into it.”
— Friedrich Nietzsche, “The Gay Science”
Eating is paradoxically completely normal and pretty weird at the same time, once you start to think about it. We eat other beings constantly, in order to remain ourselves. In modern Western logic, the potential oddity of this situation has been dealt with for the most part by assuming that the things we eat stop being themselves after ingestion, that they become fuel or building blocks for us.
However, deep in the detailed pages of journals such as Cell Host & Microbe and Nature Reviews Endocrinology, a profound transformation is occurring in scientific ideas about food and eating that promises to undo assumptions about the relationships between eaters and what is eaten. This transformation, which we might characterize as a shift from a “machinic” to a rather hallucinogenic model of food and its incorporation, endows foodstuffs with much more agency and potency than they ever had in the standard “fuel + building blocks” model, where they were just burned and redeployed.
Rather than mere nosh, provender or raw material, food and its components are now being investigated for communicative and informational properties and for roles in gene regulation, environment sensing, maintaining physiological boundaries and adjusting cellular metabolic programs. Food speaks, cues and signals. Bodies sense and respond in complicated processes of inner conversation only dimly intuited by conscious thought.
Eating as interlocution is a conceptual development that carries with it potentially disorienting new representations of human interiority and autonomy. It is at the same time an immensely practical development, with implications for nutrition and metabolism as sites of potential technological interventions in health and longevity.
The previous fuel + building blocks image of food implies a very particular kind of eating body: a motor built from smaller units that burns energy to move. The German physician and science popularizer Fritz Kahn captured the essence of this modern body in 1926 with his illustration “Der Mensch als Industriepalast” (“Man as Industrial Palace”), which shows the body as a chemical plant in which food is being broken down in the intestinal tract and processed through the liver to power the muscles. The interior of this body-factory contains workers spraying enzymes onto incoming food in the intestinal tract and sorting carbohydrate and protein materials into transport boxes in the mailroom of the liver, while the control rooms all reside in the brain.
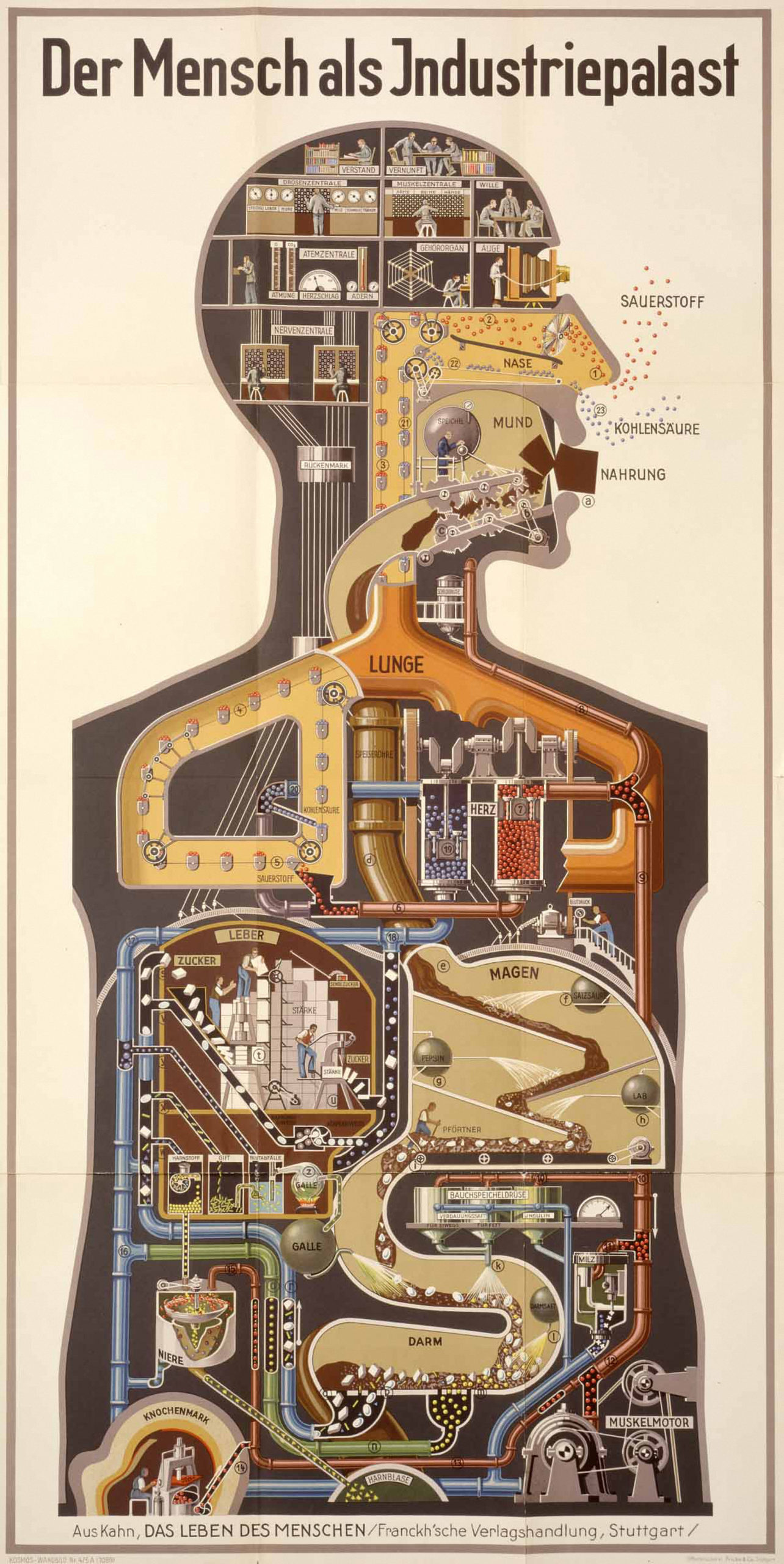
The factory envisioned by Kahn is now a hundred years behind us. What seemed the pinnacle of human progress at the time — at the height of the German chemical industry’s global dominance — reflects an industrial society based on manufacturing and the harnessing of chemical transformation for productivity. The elevation of the brain as the center of command and control — and the viscera as machine labor blindly operating as directed to process raw materials — has proved an enduring conceptual hierarchy. It adopted into the human body the idea of the capitalist class as the thinking men in the control room of the brain while the working class labored in the guts of the factory.
Is this just a metaphor, a fanciful way of communicating scientific ideas so they can be understood by lay people? Far from it. Metaphors have consequences. They shape how individuals eat, they influence how food is produced and valued, they inform public health and medical advice and, at a very fundamental level, they give form to the very categories through which we understand humans and their world.
Thus, we treat food as being without agency, as something that can be acted upon — controlled, produced, cooked, manipulated — but not something that acts in its own right with anything like its own intentionality. Even the famous saying, “you are what you eat,” originated in the chemical imagination of the 19th century: being short of a nutrient such as phosphate was seen to undermine one’s capacity for critical thinking, because the brain was rich in phosphate. The power of substrates lay only in their role as a limiting ingredient for the body.
Of course, dominant discourses always have their alternative undersides, from fresh milk advocacy to macrobiotic diets, but even the form of these minority protests — food is alive! — reveals the dominant logic of food as a blank slate only animated by its eater. Psychedelic mushrooms and this week’s trendy superfood aside, food is almost always the object, not the subject. Humans eat food. Food does not consume humans.
Or so we thought.
One direct consequence of these metaphors is a radical asymmetry in both science and medicine between what is known about topics such as genetic engineering and nutritional matters. Recently recovering from a minor surgical procedure, the physician and author Siddhartha Mukherjee mused that he was able to turn to medicine for anti-inflammatories and antibiotics targeted to his recovery from the surgical wound, but there was no good information about what he should eat to assist in the healing process.
We just haven’t done the research. We know how to engineer DNA but not what happens to the genetic material in the foods we eat. Despite living in an age of targeted molecular therapies, Mukherjee lamented, there is no science of comparable detail or sophistication able to sort out what is helpful to eat when suffering from cancer or recovering from surgery.
“Food speaks, cues and signals.”
Commentaries such as this are a sign that hundred-year-old ideas about food, eating and health are beginning to change. From the burgeoning science of the human microbiome to a sophisticated understanding of the human intestinal tract as a dense site of information exchange and sensory complexity, researchers are beginning to understand the complex conversation among foodstuffs, microbes and human cells and organs. Some scientists are beginning to ask if key actors in metabolic pathways might be targeted to control aging processes. Others are watching interchanges happening between food and body cells and asking if biotechnology can mimic the body’s molecular techniques to ferry drugs across membranes. Researchers are testing components of food, and the biochemical products that our metabolisms generate from food, as signals that could potentially be harnessed to tweak cellular energy use or tissue repair.
This presents us with a novel set of answers for the question: What is food? When we eat plants, animals and fungi, we are eating more than calories or carbohydrates, proteins, fats and vitamins. All organisms have their own cells and their own genomes. Therefore, we are eating DNA and RNA all the time. It has long been assumed that these materials are completely broken down and used as building blocks for the eater’s needs. While this is certainly the case for most consumed molecules, there are other stories unfolding at the microscopic scale of cellular interaction in the intestinal tract.
Most foodstuffs — fruit and milk, for example — contain tiny extracellular vesicles, some of which are called exosomes, made originally in the cells of, say, a grapefruit or cow. These nano-sized, fat-wrapped envelopes are particularly good at exiting and entering cells because they can cross lipid membranes and deliver their contents to the other side. The vesicles’ lipid envelopes are also protective of the transported contents through the potentially harsh territory between cells, such as in the intestinal tract. And the envelope itself can carry information about the target, a kind of address in the form of proteins on the surface that can help gain entry into certain kinds of cells but not others.
Exosomes carry many kinds of biological materials but of particular interest are microRNAs: tiny pieces of RNA that participate in gene regulation. Researchers thought for a long time that such microRNAs were active within organisms, as a kind of a tuning tool for an organism to use in controlling its own gene expression. But it turns out that vesicles from ingested food and milk can be taken up by other organisms or cells, including the bacteria that live in the gut.
Choosing ginger as an example and working with mice, a group of researchers headed by Huang-Ge Zhang at the University of Louisville recently showed that ginger-derived exosomes carrying microRNA were selectively taken up by particular microbes in the gut, in particular members of the Lactobacillaceae species, which are common, beneficial microbes in both mice and humans and therefore often included in commercial probiotics. Ginger microRNA promoted the growth of those species but also triggered changes in their gene expression. Like a tiny Rube Goldberg machine in which one unlikely contraption trips off another, the ginger-derived signal prompted the production of a bacterial enzyme that in turn signaled the mouse’s cells lining the intestine to make yet another molecule, a cytokine called IL-22.
This cytokine has the effect of promoting tissue repair. The researchers saw that its increased presence in the mucus membrane lining ameliorated symptoms from ulcers and lesions that they had intentionally induced in the mice as a model of human colitis. In short, taking in ginger microRNA was a trigger for bacterial growth and gene expression that in turn enhanced gut integrity in the host mouse.
“Humans shape their own food worlds — but more attention must be given to how those food worlds shape humans.”
This might seem head-spinning, and indeed the molecular biology behind demonstrating this set of relationships between plants, the gut microbiome and the animal host is amazingly sophisticated. No doubt there is much more to find out as this research is continued. But we can translate its implications fairly bluntly: some genetic material that is ingested is not just broken down for parts. It participates in the work of turning on and off genes in gut bacteria, whose products are relevant to the physiology of the body that eats ginger and hosts a microbiome. There is a complex interaction, mediated by molecules that we have come to understand as carrying information among the ingested food, the microbiome and the eating body itself.
Such findings are of great practical interest because gut integrity is fundamental to digestive and immune health. Having a so-called “leaky gut” allows things into the tissues that can be inflammatory or allergenic. Although the direction of the causal arrows is a little uncertain, the breakdown of the integrity of the gut wall could be both a sign and a probable promoter of autoimmune disorders and chronic inflammation. As with internal bodily barriers of all kinds — the blood-brain barrier or the placental barrier between mother and child — the ability to keep things out and yet actively and selectively transport needed substances across is fundamental to health.
Quite profound philosophical issues also arise from these findings. As with much of the new research around the human microbiome, this story raises an intriguing paradox. We need to eat other entities from outside the body in order to maintain a healthy boundary around the body. Having a detailed map of the intensive way in which apparently independent entities engage one another — through macrovesicles with tiny RNA cargo — adds another level of appreciation for what these forms of living together entail. As the recent focus on food chains as sources of novel viruses crossing from animals into humans make abundantly clear, humans shape their own food worlds — but more attention must be given to how those food worlds shape humans.

“One might well ask what taste or smell receptors are doing at such a distance from the mouth or nose.”
Signals don’t mean anything unless there is a suitable receiving apparatus. The intestinal epithelium — the layer of cells that line the digestive tract — contains scattered single hormone-producing cells with acute sensitivity to ingested nutrients and microbial metabolites. Called enteroendocrine cells, taken together these represent about 1% of the gut lining and thus rather surprisingly constitute the body’s largest — and least well-known — endocrine organ.
Think of it as an inner sensorium. Most people are used to thinking about their senses as residing at the surface of the body — eyes, ears, skin, tongue. Yet it is becoming clear that the viscera are shot through with sophisticated sensory abilities as well. Cued by what we eat, what our microbes further digest and signals from microbes, enteroendocrine cells make over 20 different hormones, most of which possess inscrutable names such as cholecystokinin and glucagon-like peptide-1. These hormones in turn act as signals to nearby cells, nerves, distant tissue and organs — and to the brain, describing what is happening in the gut and what to do about it.
Enteroendocrine hormones that most people have never heard of drive bodily sensations that everyone is familiar with. From hunger, satiety, sleepiness and nausea to more dimly felt states such as stomach acidity or intestinal motility, the drivers and responses shaping ingestion arise within an ongoing, complex chatter of sensing, signaling and calibrating.
How do enteroendocrine cells “know” and “report” the contents of the alimentary tract? They are specially equipped with receptors that are exquisitely sensitive and highly specific. Receptors are three-dimensional protein structures with high specificity for particular ligands — substances that fit into these receptors and no others, like a key into a lock, unleashing a suite of knock-on effects such as hormone production. Thus, receptors on enteroendocrine cells are “tasting” the substances descending past them and in response, are generating hormones with which to forward that information.
Some of these hormones travel a short distance to another set of receptors on the surface of nerve endings in the stomach wall and intestinal mucus membrane. These nerves in turn send a constant stream of signals — to places such as the caudal brainstem and the hypothalamus (a small region at the base of the brain) — about the volume and composition of what is eaten, even sensing detail such as the amino acid makeup of ingested protein.
One might well ask what taste or smell receptors are doing at such a distance from the mouth or nose. First discovered in the tongue and in tissue inside the nasal cavity, scientists understandably classed receptors by their apparent function in taste, smell or even light detection. But now, these receptors are turning up in places never thought of as sensory. It came as something of a surprise to find smell and taste receptors decorating the cells of tissues as diverse as kidney, lung and fat, which led to the granting of a more ecumenical name — the “chemoreceptor” — to such structures, in recognition of the more generalized role they play.
It was perhaps even more of a surprise to find that some of the chemoreceptors on the surface of the organs were specific to the byproducts of bacterial metabolism. For example, the short-chain fatty acids generated by colon bacteria act on kidney cells to trigger a rise in blood pressure, presumably because a more rapid circulation of the blood is needed to move nutrients around the body after a meal.
“Here we have a glimpse of foodstuffs as communiqués constantly being consumed and interpreted by a highly perceptive visceral sensorium.”
Here again is an enormous challenge to the chemical factory model of digestion and nutrition. Ever since the Russian physiologist Ivan Pavlov trained dogs to salivate at the sound of a bell at the turn of the 20th century, we have understood the sense-detection of food as a mental trigger for hormonal responses, which then spur the release of digestive enzymes and other elements of food’s incorporation. A cognizing, perceptive apparatus (mind) tells a dumb, blind interior (body) what to do, and the whole factory keeps running. Rethinking our guts and visceral organs as sites of sensation and communicative (if non-conscious) intelligence requires that we shed such assumptions.
While it might be tempting to replace one machine metaphor with another — say, engines with computers — what these new scientific stories tell us has the potential to undermine the whole class of metaphors: This is not a machine. If we understand eating as a form of living biochemical speech, here we have a glimpse of foodstuffs as communiqués constantly being consumed and interpreted by a highly perceptive visceral sensorium.
If our food is telling us something, what is it? Some of this conversation is about content or volume, but there may be even more interesting functions to the body’s constant interpretation of the chemistry of its food. Some plant metabolites, for example, are only produced when the plant experiences environmental stressors, like water shortage. If eaten, these plant metabolites can act as signals to cells to favor some metabolic pathways over others.
Is it possible that we have evolved to sense the larger state of the environment through the biology of the organisms we eat and to tune our metabolisms accordingly? On the practical front, is it possible to harness these signals to intentionally foster pathways conducive to metabolic health and longevity? As an aging population faces an unprecedented amount of metabolic disorder, these are pressing questions for individuals and societies.
Gaining a new respect for foods and metabolic processes does not mean rushing out to buy the latest probiotic celery juice or deciding to go on a keto diet. Well over a hundred years of pushing food down the status hierarchy, and demoting the viscera while elevating the brain, means that the kind of science described above is only the beginning of a revolution in resetting the relationships between food and health.
As recent controversy over repurposing the diabetes drug Metformin as anti-aging medication indicates, the domain of metabolic therapy is characterized more by its gaps than its certainties. Metformin — originally derived from plants in the 1920s — has been in clinical use since the 1950s and is currently the world’s most-prescribed diabetes medication. It lowers glucose production in the liver, which is useful in treating Type 2 diabetes. It has also shown longevity effects in experiments with model organisms like mice, sparking non-diabetic adults to begin taking Metformin in off-label fashion as an anti-aging therapy.
But recently, studies of non-diabetic adults showed that Metformin suppressed the effects of exercise training, reducing the gains in mitochondrial respiration in muscle cells that normally accompany increased exercise. In other words, one form of longevity-associated metabolic therapy (Metformin) might work at cross-purposes to another form (exercise). That so much remains unknown about this widely used drug shows the extent to which metabolic strategies and mechanisms have lain fallow as research areas in prior decades, particularly when considered in relation to fields such as cancer genetics and neuroscience.
“It is time to recognize the biological potency and agency of food as a metabolic partner rather than a dumb substrate.”
These new worlds — food-body crosstalk and metabolism as a domain of signals and sensing — present many conceptual and technical opportunities. Under the new model of eating as interlocution, food is being reconceived as a currency of communication between the different cells — bacterial and human — that make up the body of the eater. This chemical chatter between food, microbes, intestines, brains and nerves constitutes a different picture of what it is to know what you are eating. It also raises the question of who is involved in the conversation, since, whether we like it or not, these exquisitely complicated evolutionarily honed systems of cross-kingdom communication are now embedded in the industrialized food worlds.
It is time to recognize the biological potency and agency of food as a metabolic partner rather than a dumb substrate. It is time to push further into the still unclear but vital relationships between metabolism, aging, sleep and immunity. In doing so, we will expand our understanding of what it means to feed humanity.





